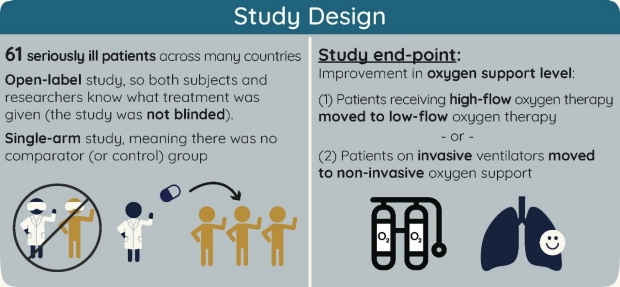
41 open-label meaning
Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc. Understanding Clinical Trial Terminology: What is a Long-Term Extension ... There is another type of study that exists between the traditional clinical trial phases and application for approval of a new medication: an open-label extension (OLE) study, sometimes also called a long-term extension study.
Open-label - definition of open-label by The Free Dictionary Define open-label. open-label synonyms, open-label pronunciation, open-label translation, English dictionary definition of open-label. adj. Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a...
Open-label meaning
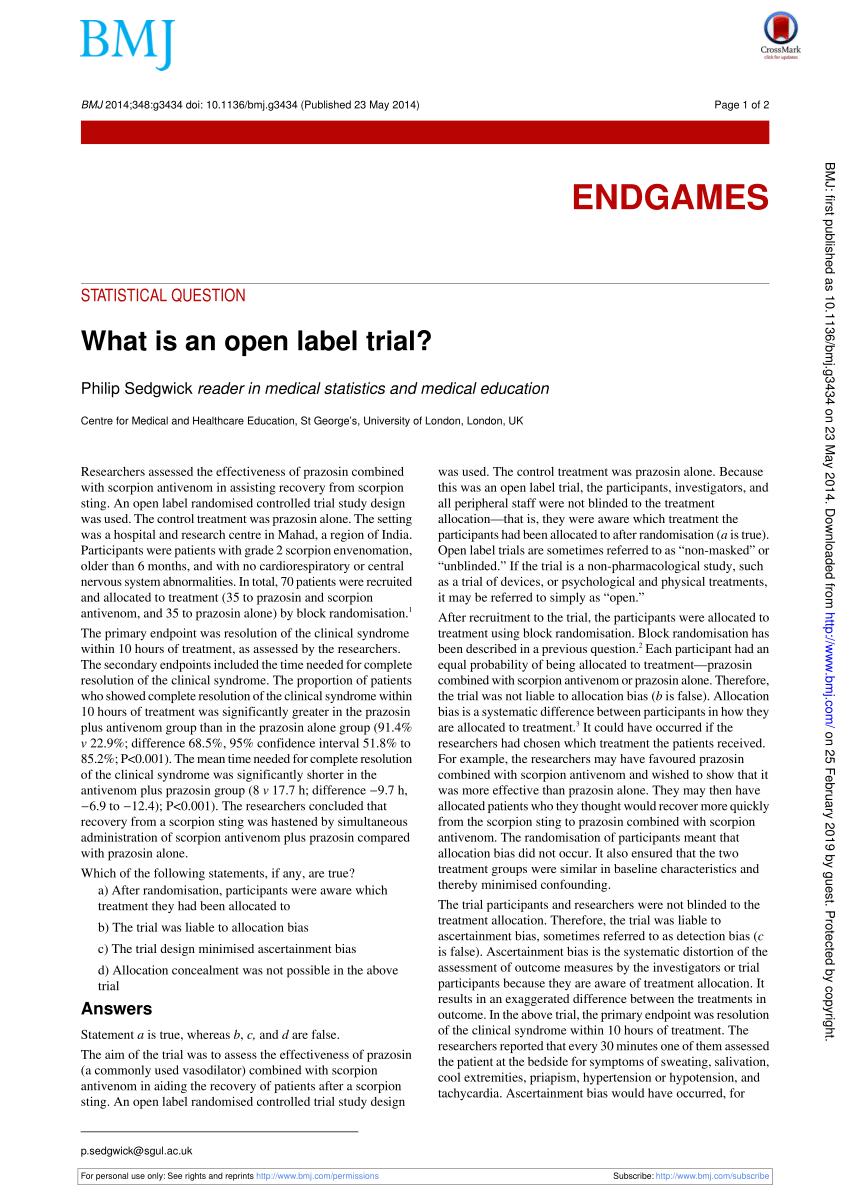
Open-label | definition of open-label by Medical dictionary open-label (ō′pən-lā′bəl) adj. Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a placebo. The American Heritage® Medical Dictionary Copyright © 2007, 2004 by Houghton Mifflin Company. Published by Houghton Mifflin Company. All rights reserved. What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities. Long-term inhaled treprostinil for PH-ILD: INCREASE open-label ... The open-label extension (OLE) evaluated long-term effects of inhaled treprostinil in PH-ILD. Methods Of 258 eligible patients, 242 enrolled in the OLE and received inhaled treprostinil. ... the mean 6MWD at week 52 was 279.1 m and the median change from RCT baseline was 3.5 m (22.1 m for the prior inhaled treprostinil arm, −19.5 m for the ...
Open-label meaning. Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] Effects of open-label placebos in clinical trials: a ... - Nature Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and analyze the ... Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. Open-Label Trial | NIH - HIV.gov Open-Label Trial A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Related Term (s) Clinical Trial Double-Blind Study
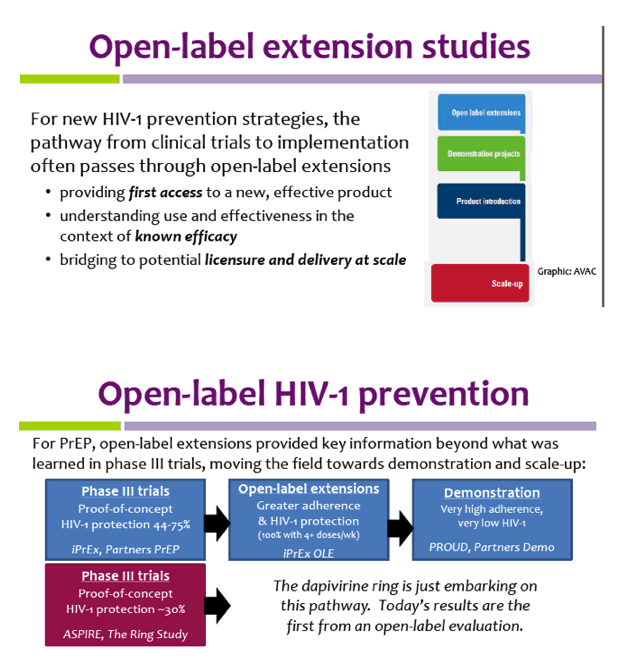
(PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants... Label Definition & Meaning - Merriam-Webster label: [verb] to affix a label to. to describe or designate with or as if with a label. Facebook - National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine. Open-label extension studies: do they provide meaningful ... - PubMed Open-label extension studies: do they provide meaningful information on the safety of new drugs? The number of open-label extension studies being performed has increased enormously in recent years. Often it is difficult to differentiate between these extension studies and the double-blind, controlled studies that preceded them.
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are used to compare different treatments or gather further information on the long-term effects of new drugs and treatments. In some instances, patients are eligible to continue... End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time. PDF Blinding Sponsors for Open Label Studies: Challenges and Solutions - MWSUG In open label studies, in addition to treatment assignment data itself, many other data elements in the CRF data can reveal treatment assignment. Those data elements include (but not limited to): A common variable that is collected for all treatment arms but takes different values for different treatment arms. Medical Definition of Open-label - MedicineNet Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. CONTINUE SCROLLING OR CLICK HERE SLIDESHOW
Open-label Definition & Meaning | YourDictionary Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a placebo. American Heritage Medicine Advertisement Find similar words to open-label using the buttons below.
What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
Open-label Definition & Meaning - Merriam-Webster open-label adjective : being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject long-term open-label studies compare double-blind, single-blind Last Updated: 29 Mar 2023 - Updated example sentences Love words? Need even more definitions?
Long-term inhaled treprostinil for PH-ILD: INCREASE open-label ... The open-label extension (OLE) evaluated long-term effects of inhaled treprostinil in PH-ILD. Methods Of 258 eligible patients, 242 enrolled in the OLE and received inhaled treprostinil. ... the mean 6MWD at week 52 was 279.1 m and the median change from RCT baseline was 3.5 m (22.1 m for the prior inhaled treprostinil arm, −19.5 m for the ...
What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities.
Open-label | definition of open-label by Medical dictionary open-label (ō′pən-lā′bəl) adj. Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a placebo. The American Heritage® Medical Dictionary Copyright © 2007, 2004 by Houghton Mifflin Company. Published by Houghton Mifflin Company. All rights reserved.





:max_bytes(150000):strip_icc()/netting.asp_v2-23d5e3c89eb24f0b817b16489bd7feed.png)















Post a Comment for "41 open-label meaning"