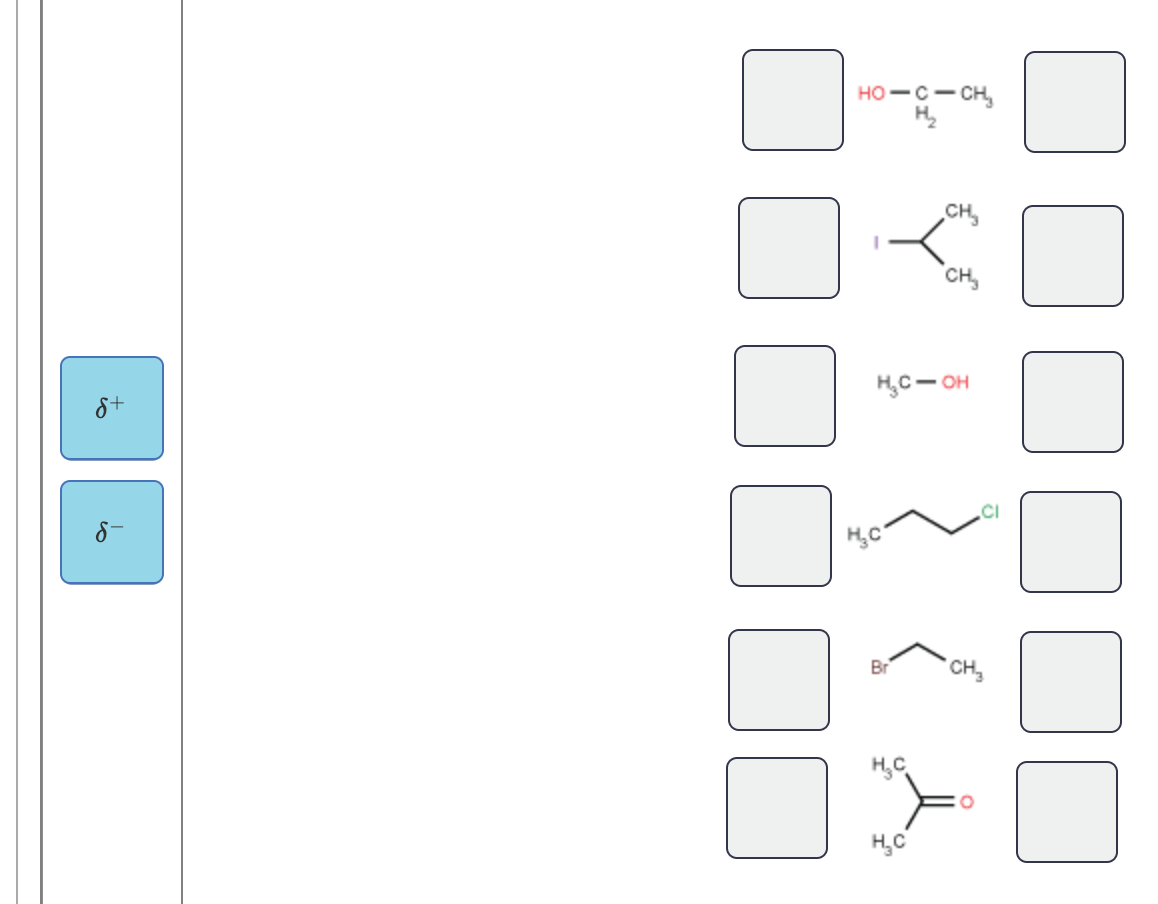
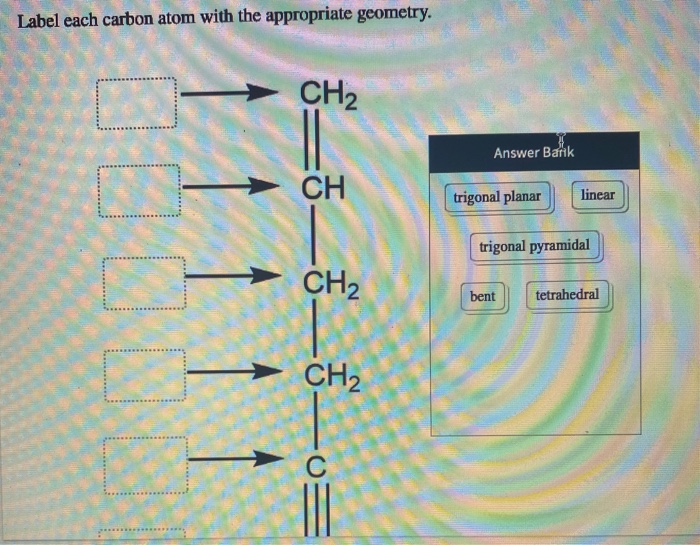
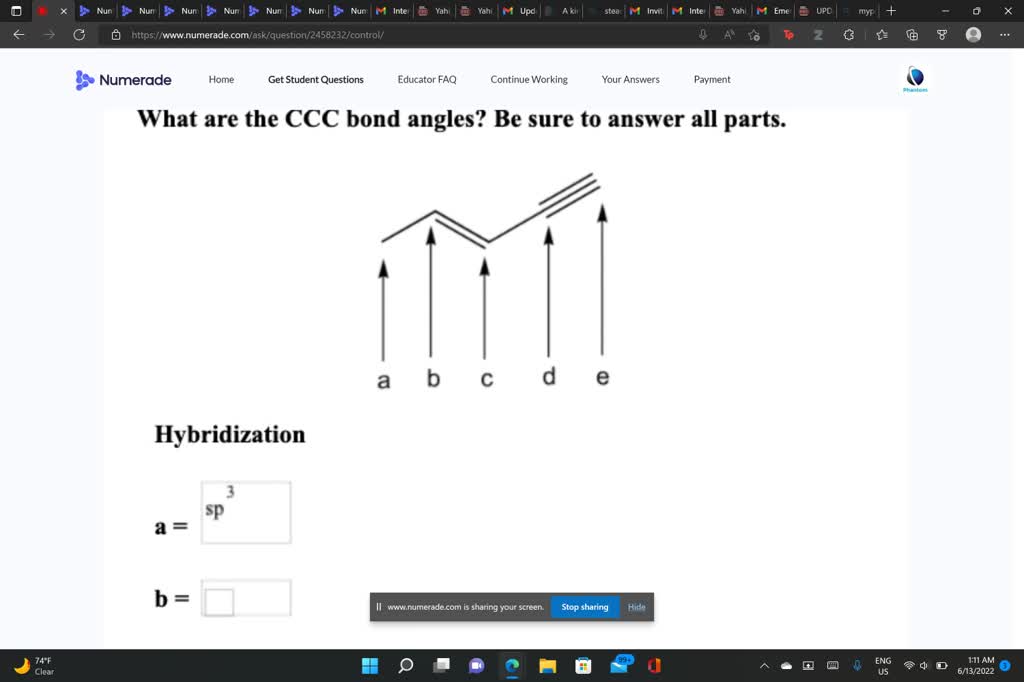
39 label each carbon atom with the appropriate geometry.
Chapter 9 Homework Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like Recall that bonds require p atomic orbitals, so the maximum hybridization of a C atom involved in a double bond is sp2 and in a triple bond is sp. There are 6 C atoms in the molecule. Starting on the left, the hybridizations are: sp2, sp2, sp3, sp, sp, sp3. All single bonds are bonds. Double and triple bonds each contain 1 bond ... 5.3: Hybridization of Atomic Orbitals - Chemistry LibreTexts Three atomic orbitals on each carbon - the 2 s, 2 px and 2 py orbitals - combine to form three sp 2 hybrids, leaving the 2 pz orbital unhybridized. The three sp 2 hybrids are arranged with trigonal planar geometry, pointing to the three corners of an equilateral triangle, with angles of 120°between them.
Label each carbon atom with the appropriate geometry. - OneClass Label each carbon atom with the appropriate geometry. Trigonal pyrimidal Trigonal planar Tetrahedral Linear Bent CH2 (double bond) CH (single bond) CH2 (single bond) CH2 (singlebond) C (triple bond) CH
Label each carbon atom with the appropriate geometry.
Answered: Label each carbon atom with the… | bartleby A: SOLUTION: Step 1: The first highlighted carbon with triple bond is SP hybridized. The carbon-carbon…. Q: Convert each shorthand structure to a complete structure with all atoms and lone pairs drawn in. a.…. A: To draw the complete structure, draw all the bonds between the atoms and lone pairs on hetero atoms…. Label Each Carbon Atom With The Appropriate Geometry. If the carbon has one triple bond or two double bonds, then hybridization is. Make sure the carbon has hybridization if it has two double bonds. [Hint for the next step] From the hybridization of each carbon, identify the geometry. step 2 of 2. The geometry of all carbon atoms is as follows: Thus, the geometry of each carbon atom is as follows ... OChem Spring 2017 Exam 1 Flashcards | Quizlet OChem Spring 2017 Exam 1. Methane. how many carbons. write formula. Click the card to flip 👆. 1 carbon. CH₄. Click the card to flip 👆. 1 / 224.
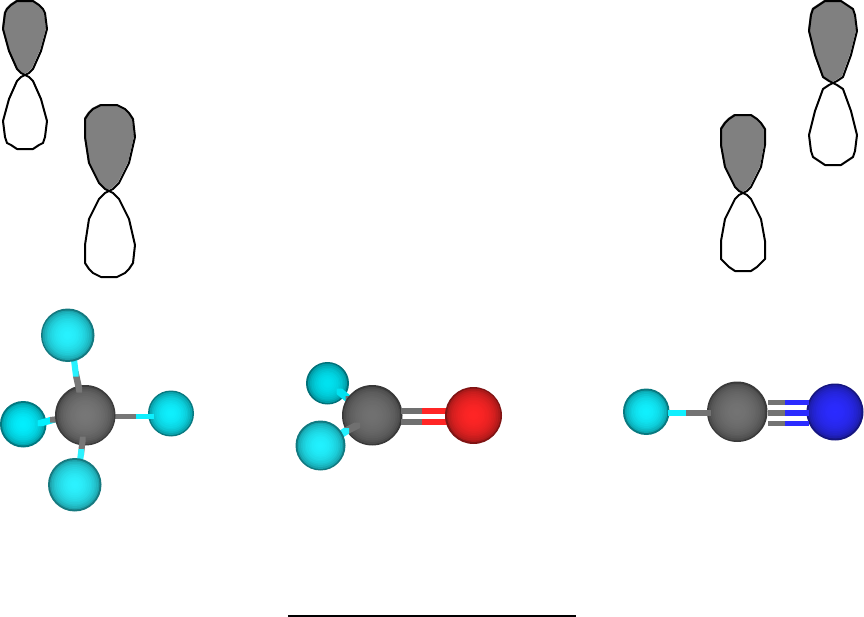
Label each carbon atom with the appropriate geometry.. [Solved] Label each carbon atom with the appropriate geometry. CH2CH ... The hybrid orbitals of carbon involve in bond formation with hydrogen. Hence, the geometry at each carbon depends on the type of hybridization. Fundamentals. The geometry of sp3 hybridized carbon atom is tetrahedral. The geometry of sp2 the hybridized carbon atom is trigonal planar. The geometry of sp hybridized carbon atom is linear. 1.3: Functional groups and organic nomenclature In an aldehyde, the carbonyl carbon is bonded on one side to a hydrogen, and on the other side to a carbon. The exception to this definition is formaldehyde, in which the carbonyl carbon has bonds to two hydrogens. A group with a carbon-nitrogen double bond is called an imine, or sometimes a Schiff base (in this book we will use the term 'imine ... 10.2: VSEPR Theory - The Five Basic Shapes - Chemistry LibreTexts 2. The carbon atom forms two double bonds. Each double bond is a group, so there are two electron groups around the central atom. Like BeH 2, the arrangement that minimizes repulsions places the groups 180° apart. 3. Once again, both groups around the central atom are bonding pairs (BP), so CO 2 is designated as AX 2. Label each carbon atom with the appropriate geometry. Bin 1 points to a ... A single C-C or C-H bond is in a tetrahedral geometry, the carbon atom is bonded to four species with a bond angle of 109°. A C=C bond is trigonal planar with a bond angle of 120°. Lastly, a C≡C bond has a linear geometry with a bond angle of 180° between the atoms of the bond.
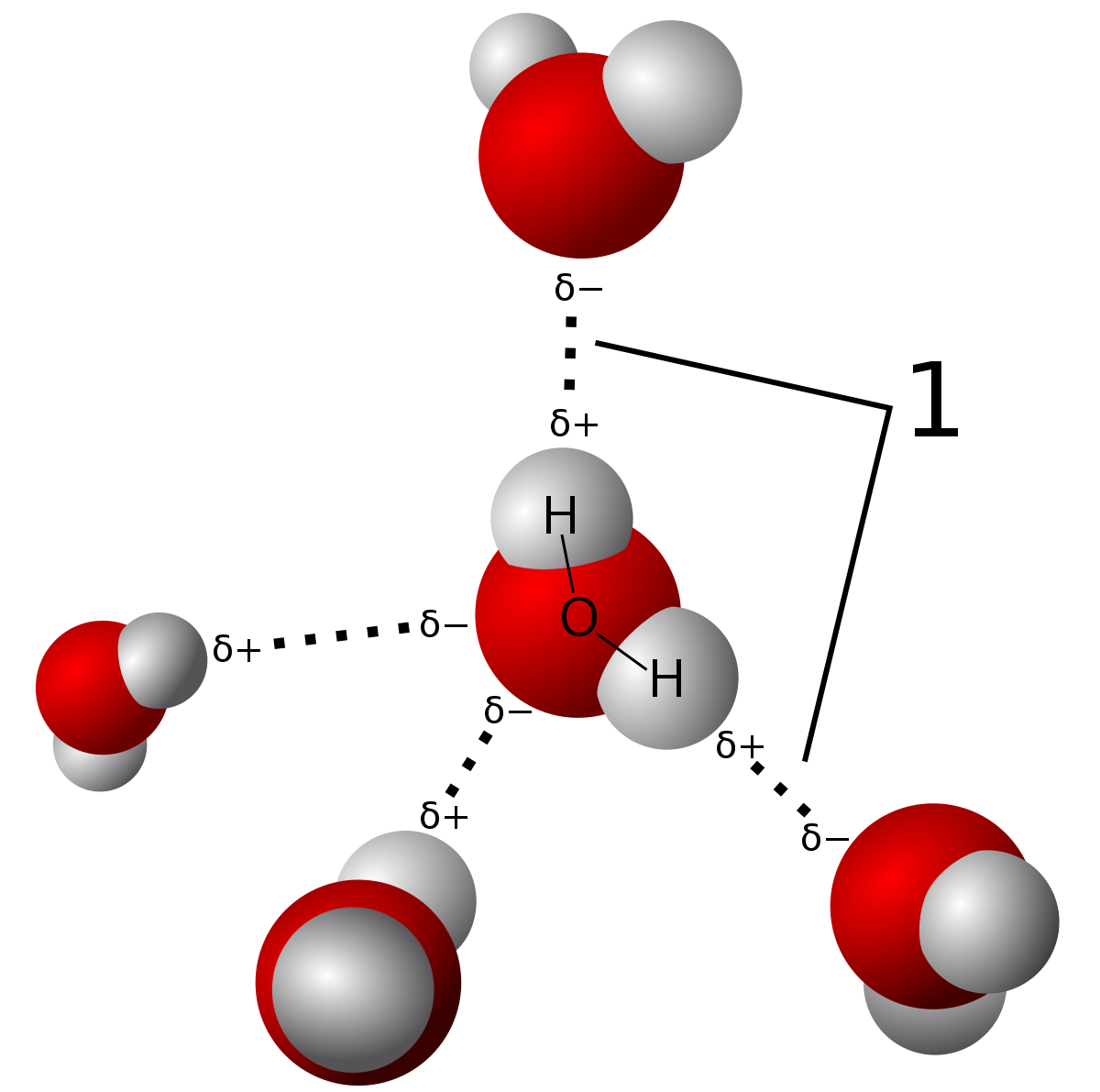
label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... 13.2: Cis-Trans Isomers (Geometric Isomers) - Chemistry LibreTexts These two compounds are cis-trans isomers (or geometric isomers), compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule. Consider the alkene with the condensed structural formula CH 3 CH=CHCH 3. We could name it 2-butene, but there are ... Bond-line structures (video) | Khan Academy Now, to do that you need to remember that a neutral carbon atom forms four bonds. So, let's see how many bonds we already have. We'll start with the carbon in magenta. The carbon in magenta already has one bond. And a neutral carbon atom forms four bonds. So, if that carbon already has one bond it needs three bonds to hydrogen. 5.10: Electronegativity and Bond Polarity - Chemistry LibreTexts Label each of the following as polar or nonpolar. Water, H 2 O: Methanol, CH 3 OH: Hydrogen Cyanide, HCN: Oxygen, O 2: Propane, C 3 H 8: Solution. Water is polar. Any molecule with lone pairs of electrons around the central atom is polar. Methanol is polar. This is not a symmetric molecule. The \(\ce{-OH}\) side is different from the other 3 ...
Question: Label each carbon atom with the appropriate geometry. - Chegg Expert Answer. 97% (87 ratings) The hybridiz …. View the full answer. Transcribed image text: Label each carbon atom with the appropriate geometry. Solved Label each carbon atom with the appropriate geometry ... - Chegg Question: Label each carbon atom with the appropriate geometry. bent trigonal planar linear trigonal pyramidal tetrahedral CH2 CH2 This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Answered: abel each carbon atom with the… | bartleby I'm not sure how I would look at the atoms in order to know what geometry they are. Transcribed Image Text: Label each carbon atom with the appropriate geometry. CH₂ §_§_§_§. CH₂ CH₂ C -I H Answer Bank trigonal planar linear bent trigonal pyramidal tetrahedral. OChem Spring 2017 Exam 1 Flashcards | Quizlet OChem Spring 2017 Exam 1. Methane. how many carbons. write formula. Click the card to flip 👆. 1 carbon. CH₄. Click the card to flip 👆. 1 / 224.
Label Each Carbon Atom With The Appropriate Geometry. If the carbon has one triple bond or two double bonds, then hybridization is. Make sure the carbon has hybridization if it has two double bonds. [Hint for the next step] From the hybridization of each carbon, identify the geometry. step 2 of 2. The geometry of all carbon atoms is as follows: Thus, the geometry of each carbon atom is as follows ...
Answered: Label each carbon atom with the… | bartleby A: SOLUTION: Step 1: The first highlighted carbon with triple bond is SP hybridized. The carbon-carbon…. Q: Convert each shorthand structure to a complete structure with all atoms and lone pairs drawn in. a.…. A: To draw the complete structure, draw all the bonds between the atoms and lone pairs on hetero atoms….
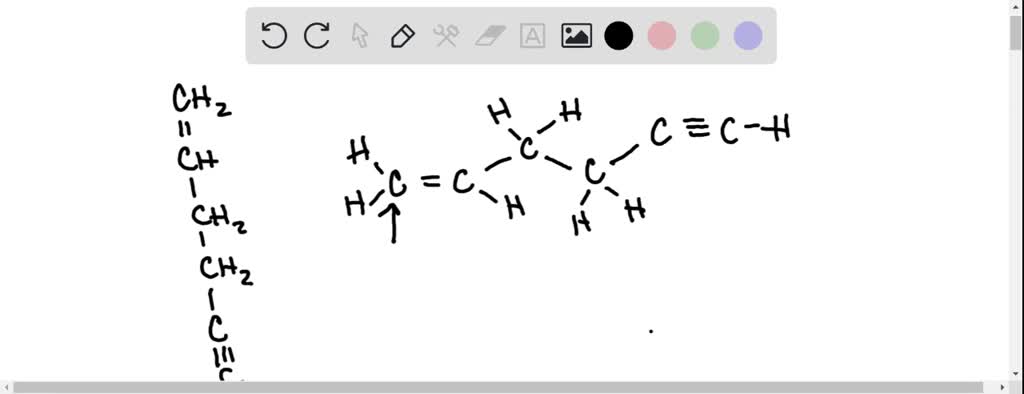
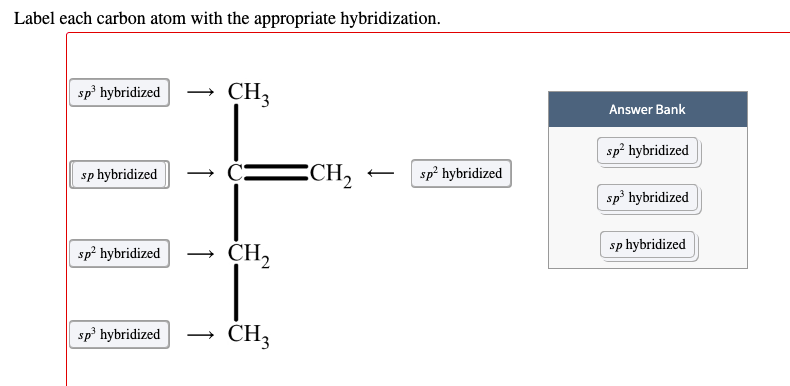
Label each carbon atom with the appropriate geometry:, CHz, Answer Bank, CH, trigonal planar, bent, CHz, trigonal pyramidal, tetrahedral, CHz, linear
Valence Bond Theory & Hybridization: Chemical Bonding Class 11 Chemistry (Ch-4) | JEE Main 2022 Exam

What are hybridisation states of each carbon atom in the following compounds? CH2 = C = O, CH3CH = CH2, (CH3)2CO, CH2 = CHCN, C6H6
























Post a Comment for "39 label each carbon atom with the appropriate geometry."